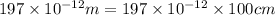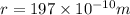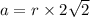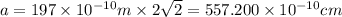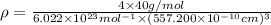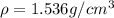Chemistry, 25.12.2019 00:31, joycetleiji1
Calcium has a cubic closest packed structure (fcc) as a solid. assuming that calcium has an atomic radius of 197 pm, calculate the density of solid calcium. (1 pm = 10-12 m, 100 cm = 1 m)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 09:00, Aminton737
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1
Do you know the correct answer?
Calcium has a cubic closest packed structure (fcc) as a solid. assuming that calcium has an atomic r...
Questions in other subjects:



Social Studies, 12.07.2019 20:00

Mathematics, 12.07.2019 20:00


Physics, 12.07.2019 20:00

Mathematics, 12.07.2019 20:00

Mathematics, 12.07.2019 20:00

History, 12.07.2019 20:00

History, 12.07.2019 20:00


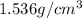 is the density of solid calcium.
is the density of solid calcium.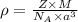
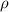 = density
= density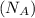 = Avogadro's number
= Avogadro's number