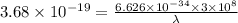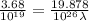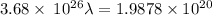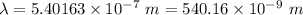Chemistry, 24.12.2019 21:31, juansantos7b
Aphoton with 2.3 ev of energy can eject an electron from potassium. what is the corresponding wavelength of this type of light? answer in nm. 1 ev = 1.60 x 10-19 j speed of light = 3.0 x 108 m/s planck's constant = 6.626 x 10-34 js socratic.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
Do you know the correct answer?
Aphoton with 2.3 ev of energy can eject an electron from potassium. what is the corresponding wavele...
Questions in other subjects:

Mathematics, 06.05.2020 06:38


Chemistry, 06.05.2020 06:38


English, 06.05.2020 06:38


English, 06.05.2020 06:38

English, 06.05.2020 06:38



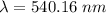
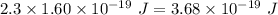
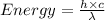
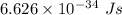
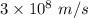
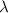 is the wavelength of the light being bombarded
is the wavelength of the light being bombarded