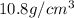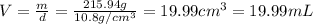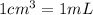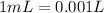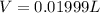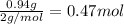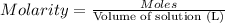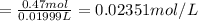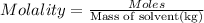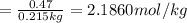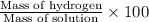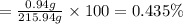Solutions of hydrogen in palladium may be formed by exposing pd metal to h gas. the concentration of hydrogen in the palladium depends on the pressure of h gas applied, but in a more complex fashion than can be described by henry’s law. under certain conditions, 0.94 g of hydrogen gas is dissolved in 215 g of palladium metal.
(a) determine the molarity of this solution (solution density = 10.8 g/cm3).
(b) determine the molality of this solution (solution density = 10.8 g/cm3).
(c) determine the percent by mass of hydrogen atoms in this solution (solution density = 10.8 g/cm3).

Answers: 1
Other questions on the subject: Chemistry



Do you know the correct answer?
Solutions of hydrogen in palladium may be formed by exposing pd metal to h gas. the concentration of...
Questions in other subjects:

Chemistry, 14.04.2021 17:10


Mathematics, 14.04.2021 17:10

Mathematics, 14.04.2021 17:10

Biology, 14.04.2021 17:10


Chemistry, 14.04.2021 17:10

Mathematics, 14.04.2021 17:10

Mathematics, 14.04.2021 17:10