
Chemistry, 24.12.2019 19:31, jeifetz1023
What is the theoretical yield of aluminum that can be produced by the reaction of 60.0 g of aluminum oxide with 30.0 g of carbon according to the following chemical equation? al2o3 + 3c → 2al + 3co

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
Do you know the correct answer?
What is the theoretical yield of aluminum that can be produced by the reaction of 60.0 g of aluminum...
Questions in other subjects:


Health, 18.09.2019 12:50


Social Studies, 18.09.2019 12:50

History, 18.09.2019 12:50

Mathematics, 18.09.2019 12:50

Health, 18.09.2019 12:50


Social Studies, 18.09.2019 12:50

Biology, 18.09.2019 12:50


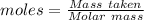
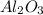
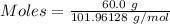
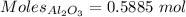
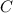
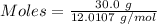
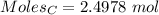
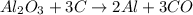
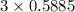 moles of carbon
moles of carbon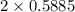 moles of aluminium.
moles of aluminium.



