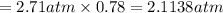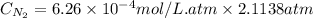As a scuba diver descends under water, the pressure increases. at a total air pressure of 2.71 atm and a temperature of 25.0 c, what is the solubility of n2 in a diver's blood? [use the value of the henry's law constant k calculated , 6.26 x 10^{-4} (mol/(l*atm).]assume that the composition of the air in the tank is the same as on land and that all of the dissolved nitrogen remains in the blood. express your answer with the appropriate units.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1


Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1
Do you know the correct answer?
As a scuba diver descends under water, the pressure increases. at a total air pressure of 2.71 atm a...
Questions in other subjects:


Chemistry, 06.06.2020 01:03

Chemistry, 06.06.2020 01:03

Mathematics, 06.06.2020 01:03

Mathematics, 06.06.2020 01:03



Mathematics, 06.06.2020 01:03


Mathematics, 06.06.2020 01:03


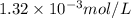 is the solubility of nitrogen gas in a diver's blood.
is the solubility of nitrogen gas in a diver's blood.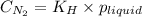
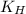 = Henry's constant =
= Henry's constant = 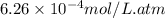
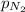 = partial pressure of nitrogen
= partial pressure of nitrogen 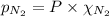 (Raoult's law)
(Raoult's law)