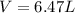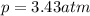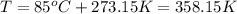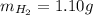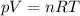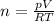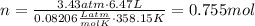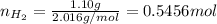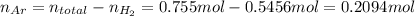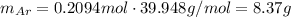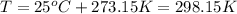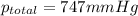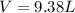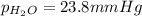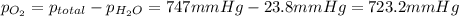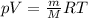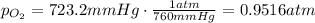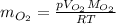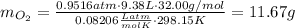Chemistry, 24.12.2019 18:31, yousifgorgees101
Amixture of hydrogen and argon gases is maintained in a 6.47 l flask at a pressure of 3.43 atm and a temperature of 85 °c. if the gas mixture contains 1.10 grams of hydrogen, the number of grams of argon in the mixture is g.
b) oxygen gas can be prepared by heating potassium chlorate according to the following equation:
2kclo3(s) > 2kcl(s) + 3o2(g)
the product gas, o2, is collected over water at a temperature of 25 °c and a pressure of 747 mm hg. if the wet o2 gas formed occupies a volume of 9.38l, the number of grams of o2 formed is g. the vapor pressure of water is 23.8 mm hg at 25 °c.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1


Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
Do you know the correct answer?
Amixture of hydrogen and argon gases is maintained in a 6.47 l flask at a pressure of 3.43 atm and a...
Questions in other subjects:

Biology, 05.05.2020 03:43

History, 05.05.2020 03:43

Mathematics, 05.05.2020 03:43



Mathematics, 05.05.2020 03:43


Mathematics, 05.05.2020 03:43

Geography, 05.05.2020 03:43

Mathematics, 05.05.2020 03:43