Chemistry, 24.12.2019 18:31, ilovewaffles70
Astudent obtains a 10.0g sample of a white powder labeled as bacl2. after completely dissolving the powder in 50.0ml of distilled water, the student adds excess na2so4(s), which causes a precipitate of baso4(s) to form, as represented by the equation above. the student filters the baso4(s), rinses it, and dries it until its mass is constant. which of the following scientific questions could best be answered based on the results of the experiment?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
Do you know the correct answer?
Astudent obtains a 10.0g sample of a white powder labeled as bacl2. after completely dissolving the...
Questions in other subjects:

Biology, 08.11.2019 22:31

Mathematics, 08.11.2019 22:31







Biology, 08.11.2019 22:31


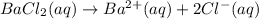
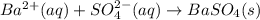

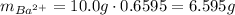 of barium cations.
of barium cations.