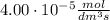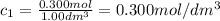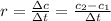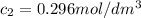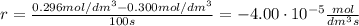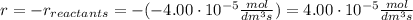Chemistry, 24.12.2019 18:31, hesterkl1225
At the start of a reaction, a 1.00 dm solution contains 0.300 mol of ethanol.
after 100 seconds the concentration of the ethanol has decreased to 0.296 mol/dmº
what is the rate of reaction over the first 100 seconds?
a 2.96 x 10-3 mol/dm/s
b 3.00 x 10 mol/dm/s
c 4.00 x 10 mol/dm®/s
d 8.00 x 10 mol/dm/s

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, RedDemon59
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
Do you know the correct answer?
At the start of a reaction, a 1.00 dm solution contains 0.300 mol of ethanol.
after 100 seconds...
after 100 seconds...
Questions in other subjects:


Mathematics, 05.05.2020 22:44



Mathematics, 05.05.2020 22:44


Mathematics, 05.05.2020 22:44



Spanish, 05.05.2020 22:44