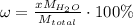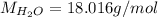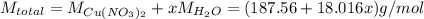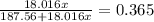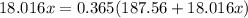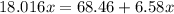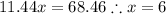Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
Do you know the correct answer?
The formula for hydrated copper(ii) nitrate is cu(no3)2.xh2o. it contains 36.5% water crystallizatio...
Questions in other subjects:



Social Studies, 12.03.2021 22:20

History, 12.03.2021 22:20



English, 12.03.2021 22:20


Biology, 12.03.2021 22:20

Mathematics, 12.03.2021 22:20