Chemistry, 24.12.2019 03:31, jaleewoodyard1
An analytical chemist is titrating 179.5ml of 0.8600ma solution of cyanic acid(hcno) with0.6500m a solution of naoh. the pka of cyanic acid is 3.46 . calculate the ph of the acid solution after the chemist has added 274.6ml of naoh the solution to it.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, Lesquirrel
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Do you know the correct answer?
An analytical chemist is titrating 179.5ml of 0.8600ma solution of cyanic acid(hcno) with0.6500m a s...
Questions in other subjects:

Social Studies, 20.05.2020 17:59

Chemistry, 20.05.2020 17:59

History, 20.05.2020 17:59


Social Studies, 20.05.2020 17:59


Mathematics, 20.05.2020 17:59

Mathematics, 20.05.2020 17:59

Biology, 20.05.2020 17:59

Chemistry, 20.05.2020 17:59


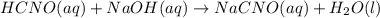
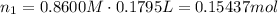
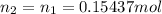
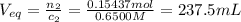

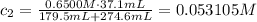
![pOH = -log[NaOH] = -log(0.053105) = 1.27](/tpl/images/0431/2587/d1ce5.png)