
Chemistry, 24.12.2019 02:31, yasminothman02
Calculate the standard enthalpy change for the reaction at 25 ∘ c. standard enthalpy of formation values can be found in this list of thermodynamic properties. c3h8(g)+ 5o2(g) ⟶ 3co2(g)+ 4h2o(g)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, anarosa331hotmailcom
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3
Do you know the correct answer?
Calculate the standard enthalpy change for the reaction at 25 ∘ c. standard enthalpy of formation va...
Questions in other subjects:


Biology, 19.04.2021 14:20


Mathematics, 19.04.2021 14:20


Physics, 19.04.2021 14:20

History, 19.04.2021 14:20

Social Studies, 19.04.2021 14:20


English, 19.04.2021 14:30


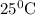 is -2043.999kJ
is -2043.999kJ ) for the given reaction is expressed as:
) for the given reaction is expressed as:![\Delta H_{rxn}^{0}=[3mol\times \Delta H_{f}^{0}(CO_{2})_{g}]+[4mol\times \Delta H_{f}^{0}(H_{2}O)_{g}]-[1mol\times \Delta H_{f}^{0}(C_{3}H_{8})_{g}]-[5mol\times \Delta H_{f}^{0}(O_{2})_{g}]](/tpl/images/0431/1998/e8c6b.png)
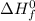 refers standard enthalpy of formation
refers standard enthalpy of formation![\Delta H_{rxn}^{0}=[3mol\times (-393.509kJ/mol)]+[4mol\times (-241.818kJ/mol)]-[1mol\times (-103.8kJ/mol)]-[5mol\times (0kJ/mol)]=-2043.999kJ](/tpl/images/0431/1998/3d0a5.png)




