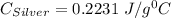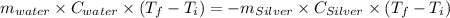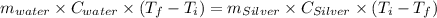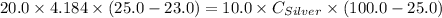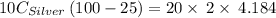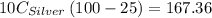Chemistry, 24.12.2019 02:31, markipler01
A10.0 gram sample of silver is heated to 100.0 degree c and then added to 20.0 g of water at 23.0 c, in an insulated container. at thermal equilibrium, the temperature of the system was measured and found to be 25.0 c. what is the specific heat, cs, of silver?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 23:00, autumperry3599
What is the chemical formula for dihydrogen monoxide
Answers: 2
Do you know the correct answer?
A10.0 gram sample of silver is heated to 100.0 degree c and then added to 20.0 g of water at 23.0 c,...
Questions in other subjects:

Spanish, 12.11.2020 01:00

Physics, 12.11.2020 01:00


Mathematics, 12.11.2020 01:00



Mathematics, 12.11.2020 01:00

Arts, 12.11.2020 01:00


History, 12.11.2020 01:00