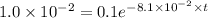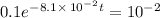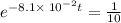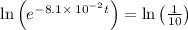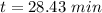Chemistry, 24.12.2019 00:31, desotoaustin
It is found that a gas undergoes a first-order decomposition reaction. if the rate constant for this reaction is 8.1 x 10-2 /min, how long will it take for the concentration of the gas to change from an initial concentration of .1m to 1.0 x 10-2 m?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ElizabethF
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
Do you know the correct answer?
It is found that a gas undergoes a first-order decomposition reaction. if the rate constant for this...
Questions in other subjects:

Physics, 07.12.2021 21:30

Mathematics, 07.12.2021 21:30

Biology, 07.12.2021 21:30

Mathematics, 07.12.2021 21:30



Mathematics, 07.12.2021 21:30

Physics, 07.12.2021 21:30

Biology, 07.12.2021 21:30


![[A_t]=[A_0]e^{-kt}](/tpl/images/0431/0820/1ef89.png)
![[A_t]](/tpl/images/0431/0820/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0431/0820/9a686.png) is the initial concentration
is the initial concentration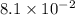 min⁻¹
min⁻¹
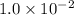 M
M