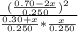Suppose a 250. ml flask is filled with 0.30 mol of n_2 and 0.70 mol of no. the following reaction becomes possible: n_2(g) +o2 → 2no(g)a. the equilibrium constant k for this reaction is 7.70 at the temperature of the flask. b. calculate the equilibrium molarity of o2. round your answer to one decimal place.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1
Do you know the correct answer?
Suppose a 250. ml flask is filled with 0.30 mol of n_2 and 0.70 mol of no. the following reaction be...
Questions in other subjects:



World Languages, 17.10.2019 05:40

Mathematics, 17.10.2019 05:40

History, 17.10.2019 05:40




Biology, 17.10.2019 05:40

Social Studies, 17.10.2019 05:40