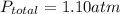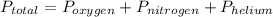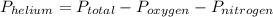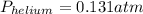Chemistry, 23.12.2019 21:31, barb4you67
Suppose that in a cylinder, the oxygen has a pressure of 0.292 atm and the nitrogen has a pressure of 0.587 atm. if the total pressure inside the cylinder is 1.01 atm, what is the pressure that is due to the helium? a. 0.722 atm b. 0.879 atm c. 0.131 atm d. 1.01 atm

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2


Chemistry, 23.06.2019 04:40, dd123984
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
Do you know the correct answer?
Suppose that in a cylinder, the oxygen has a pressure of 0.292 atm and the nitrogen has a pressure o...
Questions in other subjects:



Biology, 13.10.2020 14:01

English, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01





Physics, 13.10.2020 14:01


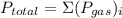 , where
, where 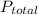 is total pressure of mixture and
is total pressure of mixture and 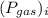 is the partial pressure of 'i'th gas.
is the partial pressure of 'i'th gas.