
Chemistry, 23.12.2019 20:31, serenityarts123
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate the work, w , if the gas expands against a constant external pressure of 1.00 atm to a final volume of 18.0 l.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, shreyapatel2004
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
Do you know the correct answer?
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate...
Questions in other subjects:

Social Studies, 24.05.2021 22:10




Mathematics, 24.05.2021 22:10

Mathematics, 24.05.2021 22:10

Mathematics, 24.05.2021 22:10


Chemistry, 24.05.2021 22:10

Mathematics, 24.05.2021 22:10


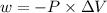
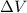 is the change in volume
is the change in volume
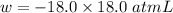
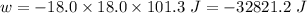 (negative sign implies that work is done by the system)
(negative sign implies that work is done by the system)



