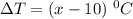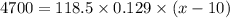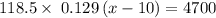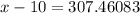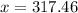Chemistry, 23.12.2019 18:31, someone2301
118.5 g piece of lead is heated with 4,700 j of energy. if the specific heat of lead is 0.129 j/ (g ⋅ °c), and the lead’s initial temperature was 10 °c, what is the final temperature of the lead?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 23.06.2019 01:00, liamgreene90
The time that is taken by neptune once around the sun is called
Answers: 1

Chemistry, 23.06.2019 06:00, irenecupcake4348
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2
Do you know the correct answer?
118.5 g piece of lead is heated with 4,700 j of energy. if the specific heat of lead is 0.129 j/ (g...
Questions in other subjects:


Social Studies, 21.05.2020 01:58



Mathematics, 21.05.2020 01:58

Chemistry, 21.05.2020 01:58


History, 21.05.2020 01:58


Mathematics, 21.05.2020 01:58


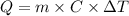
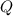 is the heat absorbed/released
is the heat absorbed/released is the temperature change
is the temperature change