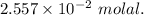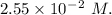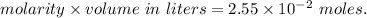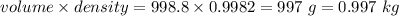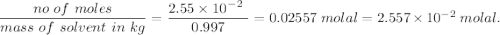Chemistry, 21.12.2019 06:31, genyjoannerubiera
A2.550 x 10^−2 m solution of glycerol (c3h8o3) in water is at 20.0°c. the sample was created by dissolving a sample of c3h8o3 in water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 998.9 ml . the density of water at 20.0°c is 0.9982 g/ml.
a. calculate the molality of the glycerol solution.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Cooldude3966
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
Do you know the correct answer?
A2.550 x 10^−2 m solution of glycerol (c3h8o3) in water is at 20.0°c. the sample was created by diss...
Questions in other subjects:

Social Studies, 11.05.2021 18:50


Mathematics, 11.05.2021 18:50


Chemistry, 11.05.2021 18:50

Mathematics, 11.05.2021 18:50

History, 11.05.2021 18:50

Mathematics, 11.05.2021 18:50

Mathematics, 11.05.2021 18:50