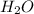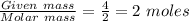Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 06:30, lwattsstudent
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1

Chemistry, 23.06.2019 17:30, dalejacksoniip5yf4y
Hydrogen-2 is also known as deuterium as well as hydrogen-3 is known as tritium hydrogen-1 is our common hydrogen isotope a sample hydrogen gas has 99% hydrogen -1 ,0.8% deuterium , and 0.2% tritium what is the average atomic mass of this mixture of isotope to the thousands place
Answers: 1


Chemistry, 23.06.2019 23:40, laura52677
If 3.50 g of the unknown compound contained 0.117 mol of c and 0.233 mol of h, how many moles of oxygen, o, were in the sample? express your answer to three significant figures and include the appropriate units.
Answers: 1
Do you know the correct answer?
What mass of water can be obtained from 4.0 g of h2 and 16 g of o2? 2 h2 + o2 > 2 h2o18 g36 g54...
Questions in other subjects:



Business, 26.07.2019 11:30

History, 26.07.2019 11:30

Social Studies, 26.07.2019 11:30

Biology, 26.07.2019 11:30

Business, 26.07.2019 11:30