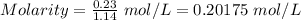Chemistry, 21.12.2019 05:31, snikergrace
At a particular temperature, k = 1.00×102 for the reaction: h2(g) + f2(g)= 2hf(g) in an experiment, at this temperature, 1.40 mol of h2 and 1.40 mol of f2 are introduced into a 1.14-l flask and allowed to react. at equilibrium, all species remain in the gas phase. what is the equilibrium concentration (in mol/l) of h2?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 04:00, Tiredd7838
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
Do you know the correct answer?
At a particular temperature, k = 1.00×102 for the reaction: h2(g) + f2(g)= 2hf(g) in an experiment,...
Questions in other subjects:

Social Studies, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Arts, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Chemistry, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01


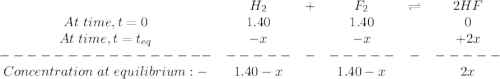
![K_c=\frac{[HF]^2}{[H_2][F_2]}](/tpl/images/0428/7279/a2854.png)
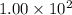
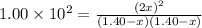

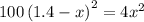

![[H_2]_{eq}=1.40-x=1.40-1.17=0.23\ moles](/tpl/images/0428/7279/ecf31.png)