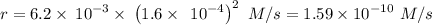The second-order rate constant for the dimerization of a protein (p) p + p → p2 is 6.2 × 10−3/m · s at 25°c. part 1 out of 2 if the concentration of the protein is 1.6 × 10−4 m, calculate the initial rate (m/s) of formation of p2. rate = × 10 m/s (enter your answer in scientific notation.)

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2

Chemistry, 23.06.2019 08:00, ira51
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
Do you know the correct answer?
The second-order rate constant for the dimerization of a protein (p) p + p → p2 is 6.2 × 10−3/m · s...
Questions in other subjects:

Business, 03.01.2020 21:31




Business, 03.01.2020 21:31






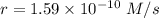
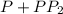
![r=k[P]^2](/tpl/images/0428/7363/45884.png)
 /Ms
/Ms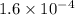 M
M