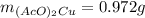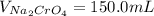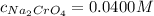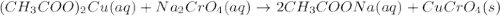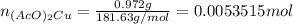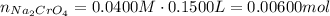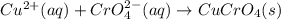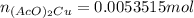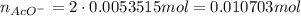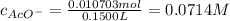Chemistry, 21.12.2019 05:31, nataliemoore1974
Suppose of copper(ii) acetate is dissolved in of a aqueous solution of sodium chromate. calculate the final molarity of acetate anion in the solution. you can assume the volume of the solution doesn't change when the copper(ii) acetate is dissolved in it. be sure your answer has the correct number of significant digits.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
Do you know the correct answer?
Suppose of copper(ii) acetate is dissolved in of a aqueous solution of sodium chromate. calculate th...
Questions in other subjects:

Mathematics, 19.08.2019 09:00




Mathematics, 19.08.2019 09:00

History, 19.08.2019 09:00


Mathematics, 19.08.2019 09:00

Mathematics, 19.08.2019 09:00