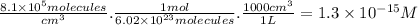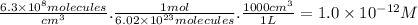The degradation of cf3ch2f (an hfc) by oh radicals in the troposphere is first order in each reactant and has a rate constant of k = 1.6 x 10^8 m^-1s^-1 at 4°c.
part a) if the tropospheric concentrations of oh and cf3ch2f are 8.1 x 10^5 and 6.3 x 10^8 molecules/cm^3, respectively, what is the rate of reaction at this temperature in m/s?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2


Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 23.06.2019 08:30, lowkstahyna
What percentage of energy used in the u. s is produced from fossil fuels
Answers: 2
Do you know the correct answer?
The degradation of cf3ch2f (an hfc) by oh radicals in the troposphere is first order in each reactan...
Questions in other subjects:

Mathematics, 08.07.2019 22:00

Mathematics, 08.07.2019 22:00

Biology, 08.07.2019 22:00




Mathematics, 08.07.2019 22:00

Social Studies, 08.07.2019 22:00


Mathematics, 08.07.2019 22:00