
Chemistry, 20.12.2019 20:31, wrightstephanie193
Agalvanic (voltaic) cell consists of an electrode composed of titanium in a 1.0 m titanium(ii) ion solution and a second electrode composed of tin in a 1.0 m tin(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25c.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2


Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 20:30, Schoolworkspace453
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
Do you know the correct answer?
Agalvanic (voltaic) cell consists of an electrode composed of titanium in a 1.0 m titanium(ii) ion s...
Questions in other subjects:

Mathematics, 16.04.2021 20:00



Mathematics, 16.04.2021 20:00

History, 16.04.2021 20:00

Physics, 16.04.2021 20:00





![E^0_{[Sn^{2+}/Sn]}=-0.14V](/tpl/images/0428/1023/81a51.png)
![E^0_{[Ti^{2+}/Ti]}=-1.63V](/tpl/images/0428/1023/456c4.png)
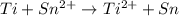
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Ti^{2+}]}{[Sn^{2+}]}](/tpl/images/0428/1023/a72a7.png)
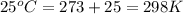

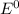 are standard reduction potentials.
are standard reduction potentials.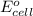 = standard electrode potential of the cell = 1.49 V
= standard electrode potential of the cell = 1.49 V = emf of the cell = ?
= emf of the cell = ?





