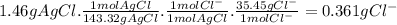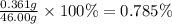To measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.1600 m silver nitrate (agno3) solution to a 46.00 g sample of the fluid and collects the solid silver chloride (agcl) product. when no more agcl is produced, he filters, washes and weighs it, and finds that 1.46 g has been produced.
the balanced chemical equation for the reaction is:
cl^- (aq) + agno3(aq) > agcl(s) + no3^- (aq)
1. what kind of reaction is this?
o precipitation o acid-base o redox
2. if you said this was a precipitation reaction, enter the chemical formula of the precipitate.
3. if you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base.
4. if you said this was a redox reaction, enter the chemical symbol of the element that is oxidized.
5. calculate the mass percent of cl in the sample. be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry




Do you know the correct answer?
To measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.1600 m silver...
Questions in other subjects:




Mathematics, 15.01.2021 01:20

Physics, 15.01.2021 01:20


Mathematics, 15.01.2021 01:20

Mathematics, 15.01.2021 01:20