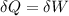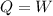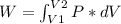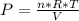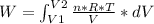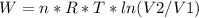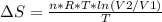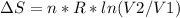Chemistry, 20.12.2019 19:31, Mattixwillard
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally reversible process.
(a) determine if the entropy change of the gas is greater than, equal to or less than zero, justify your answer
(b) determine if for the same change of state, the entropy change for an irreversible process is greater than, equal to or less than part (a)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, lindseyklewis1p56uvi
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 21.06.2019 22:30, larreanathalie3523
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
Do you know the correct answer?
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally rev...
Questions in other subjects:


History, 26.08.2020 07:01

Geography, 26.08.2020 07:01

Biology, 26.08.2020 07:01

Mathematics, 26.08.2020 07:01


Chemistry, 26.08.2020 07:01

Mathematics, 26.08.2020 07:01

Biology, 26.08.2020 07:01

Mathematics, 26.08.2020 07:01


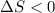
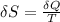
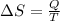
![\delta U=[tex]\delta Q- \delta W](/tpl/images/0427/9845/795a2.png)
![0=[tex]\delta Q- \delta W](/tpl/images/0427/9845/24655.png)