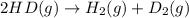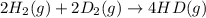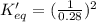The equilibrium constant is given for one of the reactions below.
determine the value o...

Chemistry, 20.12.2019 19:31, kayleedavis08
The equilibrium constant is given for one of the reactions below.
determine the value of the missing equilibrium constant.
2 hd(g) ⇌ h2(g) + d2(g) kc = 0.28
2 h2(g) + 2 d2(g) ⇌ 4 hd(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1



Do you know the correct answer?
Questions in other subjects:









Geography, 21.01.2021 22:10