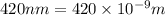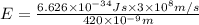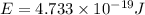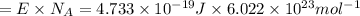Chemistry, 20.12.2019 18:31, pablogonzaleztellez
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 + hv > > > no(g) + o(g) the maximum wavelength of light that can cause this reaction is 420 nm. a) in what part of the electromagnetic spectrum is light with this wavelength found? b) what is the maximum strength of a bond, in kj/mol, that can be broken by absorption of a photon of 420-nm light? c) write out the photodissociation reaction showing lewis-dot structures.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2


Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
Do you know the correct answer?
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 +...
Questions in other subjects:

Mathematics, 16.06.2021 03:00



Mathematics, 16.06.2021 03:00


Mathematics, 16.06.2021 03:00

Business, 16.06.2021 03:00

History, 16.06.2021 03:00


Mathematics, 16.06.2021 03:00


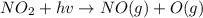
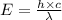
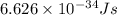
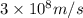
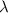 = wavelength =
= wavelength =