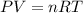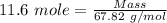Chemistry, 20.12.2019 18:31, hannabeth91
Boron trifluoride gas is collected at 2.0 degree c in an evacuated flask with a measured volume of 15.0 l. when all the gas has been collected, the pressure in the flask is measured to be 0.130 atm. calculate the mass and number of moles of boron trifluoride gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
Do you know the correct answer?
Boron trifluoride gas is collected at 2.0 degree c in an evacuated flask with a measured volume of 1...
Questions in other subjects: