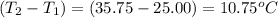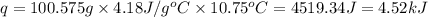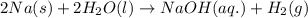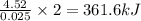Chemistry, 20.12.2019 18:31, King1Gates
Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to the chemical equation shown below.
when 0.0 25 mol of na is added to 100.00 g of water, the temperature of the resulting solution rises from 25.00°c to 35.75°c.
if the specific heat of the solution is 4.18 j/(g · °c), calculate δh for the reaction, as written.
2 na(s) + 2 h2o(l) → 2 naoh(aq) + h2(g) δh= ?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 05:00, cpcoolestkid4
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius. a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit. a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius. a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1

Chemistry, 23.06.2019 06:20, Lindsay882
Type the correct answer in each box. balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
Do you know the correct answer?
Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to the chemica...
Questions in other subjects:





English, 13.06.2020 20:57



Mathematics, 13.06.2020 20:57

History, 13.06.2020 20:57

Chemistry, 13.06.2020 20:57


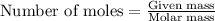
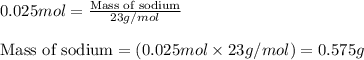
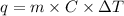
 = change in temperature =
= change in temperature =