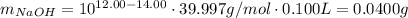Chemistry, 20.12.2019 06:31, michaellangley
Achemist must prepare of sodium hydroxide solution with a ph of at . he will do this in three steps: fill a volumetric flask about halfway with distilled water. weigh out a small amount of solid sodium hydroxide and add it to the flask. fill the flask to the mark with distilled water. calculate the mass of sodium hydroxide that the chemist must weigh out in the second step.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1


Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3

Do you know the correct answer?
Achemist must prepare of sodium hydroxide solution with a ph of at . he will do this in three steps:...
Questions in other subjects:





Mathematics, 10.03.2021 08:50


English, 10.03.2021 08:50

English, 10.03.2021 08:50

Mathematics, 10.03.2021 08:50

English, 10.03.2021 09:00


![pH = -log[H_3O^+]](/tpl/images/0427/4560/89cf7.png) .NaOH is a strong base, as it's a hydroxide formed with a group 1A metal, so it dissociates fully in water by the equation:
.NaOH is a strong base, as it's a hydroxide formed with a group 1A metal, so it dissociates fully in water by the equation:  .From the equation above, using stoichiometry we can tell that the molarity of hydroxide is equal to the molarity of NaOH:
.From the equation above, using stoichiometry we can tell that the molarity of hydroxide is equal to the molarity of NaOH: ![[NaOH] = [OH^-]](/tpl/images/0427/4560/b5cb4.png) .Concentration of hydroxide is then equal to the ratio of moles of NaOH and the volume of the given solution. Moles themselves are equal to mass over molar mass, so we obtain:
.Concentration of hydroxide is then equal to the ratio of moles of NaOH and the volume of the given solution. Moles themselves are equal to mass over molar mass, so we obtain: ![[OH^-] = [NaOH] = \frac{n_{NaOH}}{V} = \frac{m_{NaOH}}{M_{NaOH}V}](/tpl/images/0427/4560/2bd04.png) .We also know that
.We also know that ![pOH = 14.00 - pH = -log[NaOH]](/tpl/images/0427/4560/e6921.png) . Take the antilog of both sides:
. Take the antilog of both sides: ![10^{-pOH} = 10^{pH - 14.00} = [NaOH] = \frac{m_{NaOH}}{M_{NaOH}V}](/tpl/images/0427/4560/d48cb.png) .Solve for the mass of NaOH:
.Solve for the mass of NaOH:  .
.