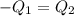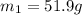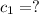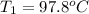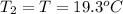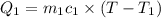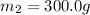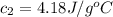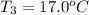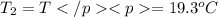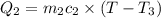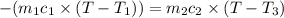Chemistry, 20.12.2019 05:31, nihadsalim10
A51.9 g sample of quartz is put into a calorimeter (see sketch at right) that contains 300.0 g of water. the quartz sample starts off at 97.8 °c and the temperature of the water starts off at 17.0 °c. when the temperature of the water stops changing it's 19.3 °c. the pressure remains constant at 1 atm. insulated container water sample calculate the specific heat capacity of quartz according to this experiment. be sure your answer is rounded to 2 significant digits. a calorimeter g °c.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2


Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Do you know the correct answer?
A51.9 g sample of quartz is put into a calorimeter (see sketch at right) that contains 300.0 g of wa...
Questions in other subjects:

Biology, 18.07.2019 09:30

Biology, 18.07.2019 09:30

Biology, 18.07.2019 09:30


History, 18.07.2019 09:30


Biology, 18.07.2019 09:30


Biology, 18.07.2019 09:30

Social Studies, 18.07.2019 09:30