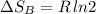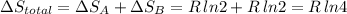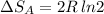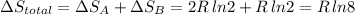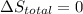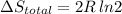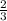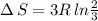Chemistry, 20.12.2019 04:31, blakesmith0110
Arigid container is divided into two compartments of equal volume by a partition. one compartment contains 1 mole of ideal gas a at 1 atm, and the other contains 1 mole of ideal gas b at 1 atm. calculate the increase in entropy which occurs when the partition between the two compartments is removed. if the frst compartment had contained 2 moles of ideal gas a, what would have been the increase in entropy when the partition was removed? calculate the corresponding increases in entropy in each of the preceding two situations if both compartments had contained ideal gas a.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Do you know the correct answer?
Arigid container is divided into two compartments of equal volume by a partition. one compartment co...
Questions in other subjects:


Mathematics, 14.04.2020 22:54


Physics, 14.04.2020 22:54


Mathematics, 14.04.2020 22:54


Mathematics, 14.04.2020 22:54



 .
.