
Chemistry, 20.12.2019 02:31, ameera1973
1.00 liter solution contains 0.40 m acetic acid and 0.52 m potassium acetate. if 0.130 moles of calcium hydroxide are added to this system, indicate whether the following statements are true or false. (assume that the volume does not change upon the addition of calcium hydroxide.)
a. the number of moles of ch3cooh will increase.
b. the number of moles of ch3coo- will decrease.
c. the equilibrium concentration of h3o will remain the same.
d. the ph will decrease.
e. the ratio of [ch3cooh] / [ch3coo-] will increase.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Do you know the correct answer?
1.00 liter solution contains 0.40 m acetic acid and 0.52 m potassium acetate. if 0.130 moles of calc...
Questions in other subjects:


History, 24.06.2020 08:01

Mathematics, 24.06.2020 08:01


History, 24.06.2020 08:01

Mathematics, 24.06.2020 08:01



English, 24.06.2020 08:01

Mathematics, 24.06.2020 08:01


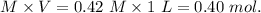
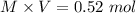
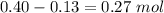
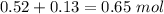
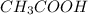 decrease and
decrease and 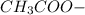 increase.
increase.



