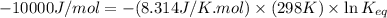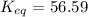Chemistry, 20.12.2019 01:31, maelaysiap
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than does oxygen (o2), as indicated by these approximate standard free-energy changes in blood:
reaction a: reaction b: hb+o2hb+co⟶⟶hbo2,hbco, δg∘=−70 kj/mol δg∘=−80 kj/mol
estimate the equilibrium constant k at 298 k for the equilibrium
hbo2+co⇌hbco+o2

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2


Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
Do you know the correct answer?
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than doe...
Questions in other subjects:

Mathematics, 20.04.2020 14:31


Computers and Technology, 20.04.2020 14:31


Physics, 20.04.2020 14:55

Geography, 20.04.2020 14:55




Chemistry, 20.04.2020 14:56


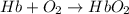 ;
; 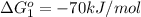
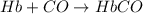 ;
; 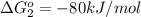
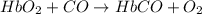 ;
; 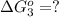
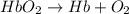 ;
; 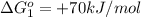
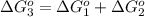
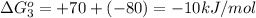
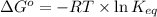
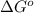 = standard Gibbs free energy = -10kJ/mol = -10000 J/mol
= standard Gibbs free energy = -10kJ/mol = -10000 J/mol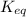 = equilibrium constant = ?
= equilibrium constant = ?