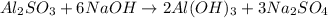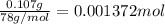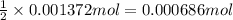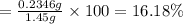Chemistry, 19.12.2019 23:31, stephaniem0216
Amixture contains only nacl and al2(so4)3. a 1.45-g sample of the mixture is dissolved in water, and an ex- cess of naoh is added, producing a precipitate of al(oh)3. the precipitate is filtered, dried, and weighed. the mass of the precipitate is 0.107 g. what is the mass percent of al2(so4)3 in the sample?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
Do you know the correct answer?
Amixture contains only nacl and al2(so4)3. a 1.45-g sample of the mixture is dissolved in water, and...
Questions in other subjects:

World Languages, 30.11.2020 17:00

Biology, 30.11.2020 17:00

History, 30.11.2020 17:00


Mathematics, 30.11.2020 17:00

Chemistry, 30.11.2020 17:00