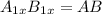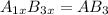Chemistry, 19.12.2019 20:31, clmorcutt420
In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrahedral holes is 1: 1: 2.
what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy all the octahedral holes? what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy all the octahedral and tetrahedral holes?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1


Chemistry, 22.06.2019 20:30, lexibyrd120
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
Do you know the correct answer?
In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrah...
Questions in other subjects:



Physics, 03.01.2020 21:31




Computers and Technology, 03.01.2020 21:31