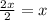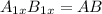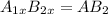In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrahedral holes is 1: 1: 2. what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy half of the tetrahedral holes? ab a3b ab2 a2b what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy all the tetrahedral holes? ab a2b ab2 a3b

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
Do you know the correct answer?
In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrah...
Questions in other subjects:





English, 31.07.2019 16:00


Mathematics, 31.07.2019 16:00


English, 31.07.2019 16:00