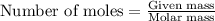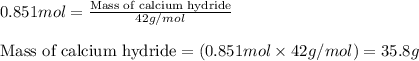Chemistry, 19.12.2019 20:31, cameronrandom00
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g)how many grams of cah2 are needed to generate 48.0 l of h2 gas at a pressure of 0.888 atm and a temperature of 32°c? calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g)how many grams of cah2 are needed to generate 48.0 l of h2 gas at a pressure of 0.888 atm and a temperature of 32°c? 35.850.70.85171.7143

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1

Chemistry, 23.06.2019 14:00, LeoInc6806
[07.06] which of the following chemical reactions is an oxidation-reduction reaction? (2 points) wo3 + 3h2 yields w + 3h2o kno3 + licl yields lino3 + kcl caso4 + 2nacl yields na2so4 + cacl2 mg(no3)2 + 2hbr yields mgbr2 + 2hno3
Answers: 1

Chemistry, 23.06.2019 15:20, KhloodAhmed
Plzzz ? which stores information in discrete steps? a magnet and coil of wire compact discs plastic records amplified speakers
Answers: 2
Do you know the correct answer?
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2...
Questions in other subjects:


Mathematics, 18.10.2019 20:40


Mathematics, 18.10.2019 20:40



Mathematics, 18.10.2019 20:40





![32^oC=[32+273]K=305K](/tpl/images/0426/4065/40af0.png)


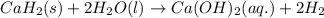
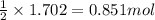 of calcium hydride
of calcium hydride