
Chemistry, 19.12.2019 20:31, harshakayla02
Ascientist measures the standard enthalpy change for the following reaction to be -190.0 kj : hcl(g) + nh3(g) nh4cl(s) based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of nh4cl(s) is kj/mol.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
Do you know the correct answer?
Ascientist measures the standard enthalpy change for the following reaction to be -190.0 kj : hcl(g...
Questions in other subjects:

Mathematics, 23.10.2019 06:00

Mathematics, 23.10.2019 06:00

History, 23.10.2019 06:00

Biology, 23.10.2019 06:00


History, 23.10.2019 06:00

Physics, 23.10.2019 06:00



Spanish, 23.10.2019 06:00


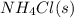 is -328.4 kJ/mol
is -328.4 kJ/mol![\Delta H_{rxn}=\sum [n\times \Delta H_f_{(products)}]-\sum [n\times \Delta H_f_{(reactants)}]](/tpl/images/0426/4202/192b9.png)
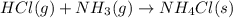
![\Delta H_{rxn}=[(1\times \Delta H_f_{(NH_4Cl(s))})]-[(1\times \Delta H_f_{(NH_3(g))})+(1\times \Delta H_f_{(HCl(g))})]](/tpl/images/0426/4202/589ac.png)
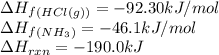
![-190.0=[(1\times \Delta H_f_{(NH_4Cl(s))})]-[(1\times (-46.1))+(1\times (-92.30))]\\\\\Delta H_f_{(NH_4Cl(s))}=-328.4kJ/mol](/tpl/images/0426/4202/9648b.png)




