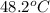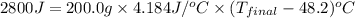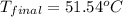Chemistry, 19.12.2019 02:31, mlarsen5000
In a coffee-cup calorimeter, 150.0 ml of 0.50 m hcl is added to 50.0 ml of 1.00 m naoh to make 200.0 g solution at an initial temperature of 48.2°c. if the enthalpy of neutralization for the reaction between a strong acid and a strong base is −56 kj/mol, calculate the final temperature of the calorimeter contents. assume the specific heat capacity of the solution is 4.184 j°c⁻¹ g⁻¹ and assume no heat loss to the surroundings.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 08:30, microwave13016
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Do you know the correct answer?
In a coffee-cup calorimeter, 150.0 ml of 0.50 m hcl is added to 50.0 ml of 1.00 m naoh to make 200.0...
Questions in other subjects:



Mathematics, 08.12.2020 03:10

Mathematics, 08.12.2020 03:10

Mathematics, 08.12.2020 03:10



Mathematics, 08.12.2020 03:10


Mathematics, 08.12.2020 03:10


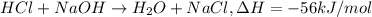
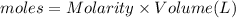
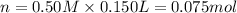
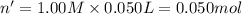
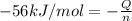
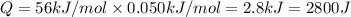
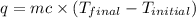
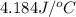
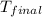 = final temperature =
= final temperature = 
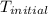 = initial temperature =
= initial temperature =