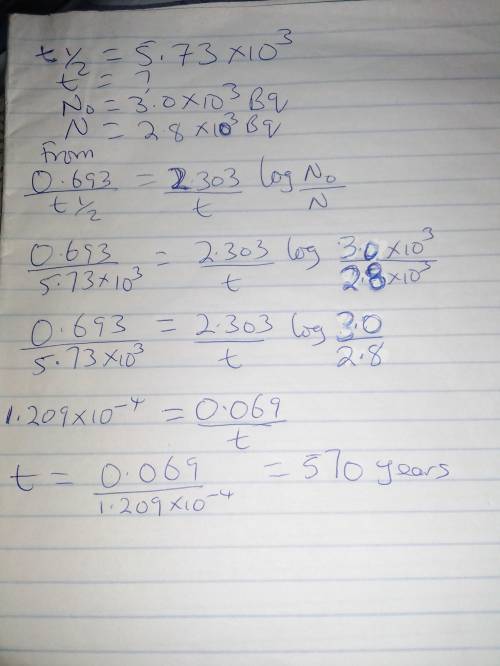Chemistry, 19.12.2019 00:31, Svetakotok
The half-life for the decay of carbon-14 is 5.73x10^3 years. suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of woodfrom an archeological dig is measured to be 2.8x10^3 bq. the activity in a similiar-sized sample of fresh wood is measured to be 3.0x10^3 bq. calculate the age of the artifact. round your answer to 2 significant digits.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 16:30, shreyapatel2004
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1
Do you know the correct answer?
The half-life for the decay of carbon-14 is 5.73x10^3 years. suppose the activity due to the radioac...
Questions in other subjects:



Mathematics, 05.05.2020 14:44



English, 05.05.2020 14:44

Physics, 05.05.2020 14:44

Mathematics, 05.05.2020 14:44

Health, 05.05.2020 14:44

English, 05.05.2020 14:44