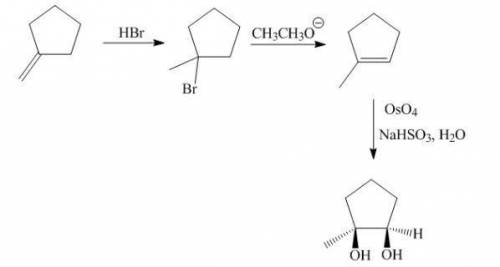
Chemistry, 18.12.2019 23:31, 4300402428
The target diol is synthesized in one step from 1-methylcyclopentene, but your lab partner exhausted the supply of that alkene. fortunately, you have plenty of isomers (c6h10) on hand from which to synthesize 1-methylcyclopentene and, ultimately, the diol. provide the missing reagents and organic structures needed to most efficiently produce the target product.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1
Do you know the correct answer?
The target diol is synthesized in one step from 1-methylcyclopentene, but your lab partner exhausted...
Questions in other subjects:


Mathematics, 18.08.2019 16:50

Biology, 18.08.2019 16:50


Mathematics, 18.08.2019 16:50




Mathematics, 18.08.2019 16:50


 .
.