
Rusting of iron is a very common chemical reaction. it results in one form from fe reacting with oxygen gas to produce iron (iii) oxide. your sample of iron is 12.0 moles of iron. so which if these is a true statement? note: all numbers located immediately after elemental symbols below should be considered subscripts. a. 4.5 moles of o2 and produce 3.0 moles of fe2o3. b. 12.0 moles of o2 and produce 24.0 moles of fe2o3. c. 9.0 moles of o2 and produce 3.0 moles of fe2o3. d. 9.0 moles of o2 and produce 6.0 moles of fe2o3 e. none of the above

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2



Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
Do you know the correct answer?
Rusting of iron is a very common chemical reaction. it results in one form from fe reacting with oxy...
Questions in other subjects:



Chemistry, 27.06.2019 00:00


Mathematics, 27.06.2019 00:00

Chemistry, 27.06.2019 00:00

Mathematics, 27.06.2019 00:00

History, 27.06.2019 00:00


Mathematics, 27.06.2019 00:00


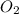 and produce 6.0 moles of
and produce 6.0 moles of 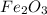
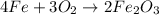
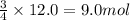 of oxygen gas
of oxygen gas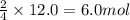 of iron (III) oxide
of iron (III) oxide



