Soda drinks bubble when the bottle is opened because:
(1) the temperature of the soda...

Soda drinks bubble when the bottle is opened because:
(1) the temperature of the soda increases.
(2) exposure to atmospheric pressure squeezes the carbon dioxide from solution.
(3) the partial pressure of carbon dioxide above the solution is reduced.
(4) atmospheric nitrogen molecules displace carbon dioxide molecules.
(5) the henry's law constant changes due to the change in pressure.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 05:30, xarianna2007
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1

Do you know the correct answer?
Questions in other subjects:

Computers and Technology, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10








Mathematics, 18.03.2021 02:10


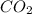 is pressurized in the bottle.
is pressurized in the bottle.



