
Chemistry, 18.12.2019 19:31, jilliynr9507
If solid nacl is added to a saturated water solution of pbcl2 at 20o c, a precipitate is formed. how would this affect the value of the ksp for [pb2+][cl-] in solution? the ksp increases. the ksp decreases. the ksp remains the same. none of the above.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1


Chemistry, 23.06.2019 02:00, hermesrobles
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
Do you know the correct answer?
If solid nacl is added to a saturated water solution of pbcl2 at 20o c, a precipitate is formed. how...
Questions in other subjects:

Biology, 25.09.2019 05:00




History, 25.09.2019 05:00


Chemistry, 25.09.2019 05:00

English, 25.09.2019 05:00


History, 25.09.2019 05:00


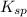 remains the same
remains the same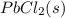 ⇄
⇄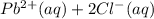
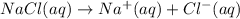
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0424/5289/7fd11.png)
![S=[Pb^{2+}]=\frac{K_{sp}}{[Cl^-]^2}](/tpl/images/0424/5289/c2c31.png)




