Chemistry, 18.12.2019 18:31, odriskel49
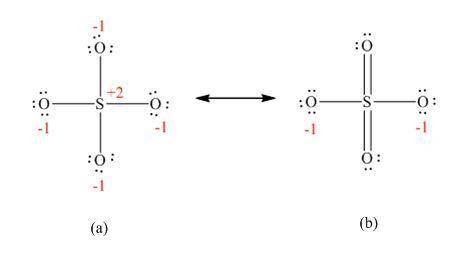
Be sure to answer all parts. the sulfate ion can be represented with four s―o bonds or with two s―o and two s═o bonds. (a) which representation is better from the standpoint of formal charges? four s―o bonds two s―o bonds and two s═o bonds (b) what shape is the sulfate ion, and what hybrid orbitals of s are postulated for the σ bonding? bent tetrahedral trigonal planar trigonal pyramidal spmdn where m = and n = (c) in view of the answer to part (b), what orbitals of s must be used for the π bonds? what orbitals of o? sulfur: 3d 3p 2p 3s oxygen: 3p 2p 2s 3s

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
Do you know the correct answer?
Be sure to answer all parts. the sulfate ion can be represented with four s―o bonds or with two s―o...
Questions in other subjects:

Mathematics, 03.07.2020 14:01


Geography, 03.07.2020 14:01





Mathematics, 03.07.2020 14:01



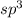 hybrid orbitals invovle sigma bonding.
hybrid orbitals invovle sigma bonding.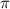 orbitals are formed by the overlapping of d-orbitals of sulfur with p-orbitals of oxygen.
orbitals are formed by the overlapping of d-orbitals of sulfur with p-orbitals of oxygen.