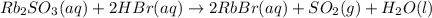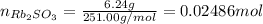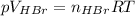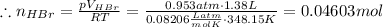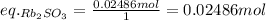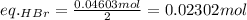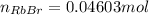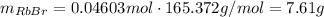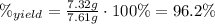Chemistry, 18.12.2019 07:31, Zachary429
Asolid sample of rb2so3 weighing 6.24 g reacts with 1.38 l gaseous hbr, measured at 75°c and 0.953 atm pressure. the solid rbbr, extracted from the reaction mixture and purified, has a mass of 7.32 g.
(a) what is the limiting reactant?
(b) what is the theoretical yield of rbbr, assuming com- plete reaction?
(c) what is the actual percentage yield of product?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
Do you know the correct answer?
Asolid sample of rb2so3 weighing 6.24 g reacts with 1.38 l gaseous hbr, measured at 75°c and 0.953 a...
Questions in other subjects:

Mathematics, 20.05.2020 21:01

Mathematics, 20.05.2020 21:01


Health, 20.05.2020 21:01

Chemistry, 20.05.2020 21:01

History, 20.05.2020 21:01


Mathematics, 20.05.2020 21:01