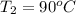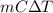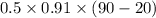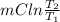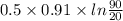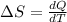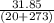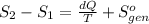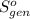Ablock of aluminum with m = 0.5 kg, t = 20oc is dropped into a reservoir at a temperature of 90oc. calculate (a) the change in stored energy (δe), (b) the amount of heat transfer (q), (c) the change in entropy (δs), (d) the amount of entropy transfer by heat and (e) the entropy generation (sgen, univ) in the system's universe during the heat transfer process.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1


Chemistry, 23.06.2019 12:50, jasonoliva13
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1

Chemistry, 23.06.2019 14:00, cfonse11
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1
Do you know the correct answer?
Ablock of aluminum with m = 0.5 kg, t = 20oc is dropped into a reservoir at a temperature of 90oc. c...
Questions in other subjects:

English, 28.12.2020 16:10

Social Studies, 28.12.2020 16:10




Physics, 28.12.2020 16:10



English, 28.12.2020 16:10


 ,
,