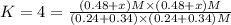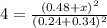Chemistry, 18.12.2019 05:31, djmelodiedaniels
When the reaction co2(g) + h2(g) ⇄ h2o(g) + co(g) is at equilibrium at 1800◦c, the equilibrium concentrations are found to be [co2] = 0.24 m, [h2] = 0.24 m, [h2o] = 0.48 m, and [co] = 0.48 m. then an additional 0.34 moles per liter of co2 and h2 are added. when the reaction comes to equilibrium again at the same temperature, what will be the molar concentration of co?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
Do you know the correct answer?
When the reaction co2(g) + h2(g) ⇄ h2o(g) + co(g) is at equilibrium at 1800◦c, the equilibrium conce...
Questions in other subjects:

Chemistry, 26.08.2019 00:30

Mathematics, 26.08.2019 00:30


Mathematics, 26.08.2019 00:30


Geography, 26.08.2019 00:30

Geography, 26.08.2019 00:30



Mathematics, 26.08.2019 00:30


![[CO_2] = 0.24 M, [H_2] = 0.24 M, [H_2O] = 0.48 M, [CO] = 0.48 M](/tpl/images/0423/7365/45c13.png)
![K=\frac{[H_2O][CO]}{[CO_2][H_2]}=\frac{0.48 M\times 0.48 M}{0.24 M\times 0.24 M}](/tpl/images/0423/7365/2a444.png)
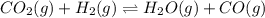
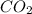 and
and 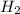 are added.
are added.